
However, sugars will only have one aldehyde OR one ketone functional group. Carbohydrates generally have multiple alcohol functional groups, so we never focus on those. Sugars, or carbohydrates, have two major functional groups: an aldehyde or a ketone (both are collectively called carbonyls), and an alcohol functional group. Naming the Major Functional Group in a Carbohydrate

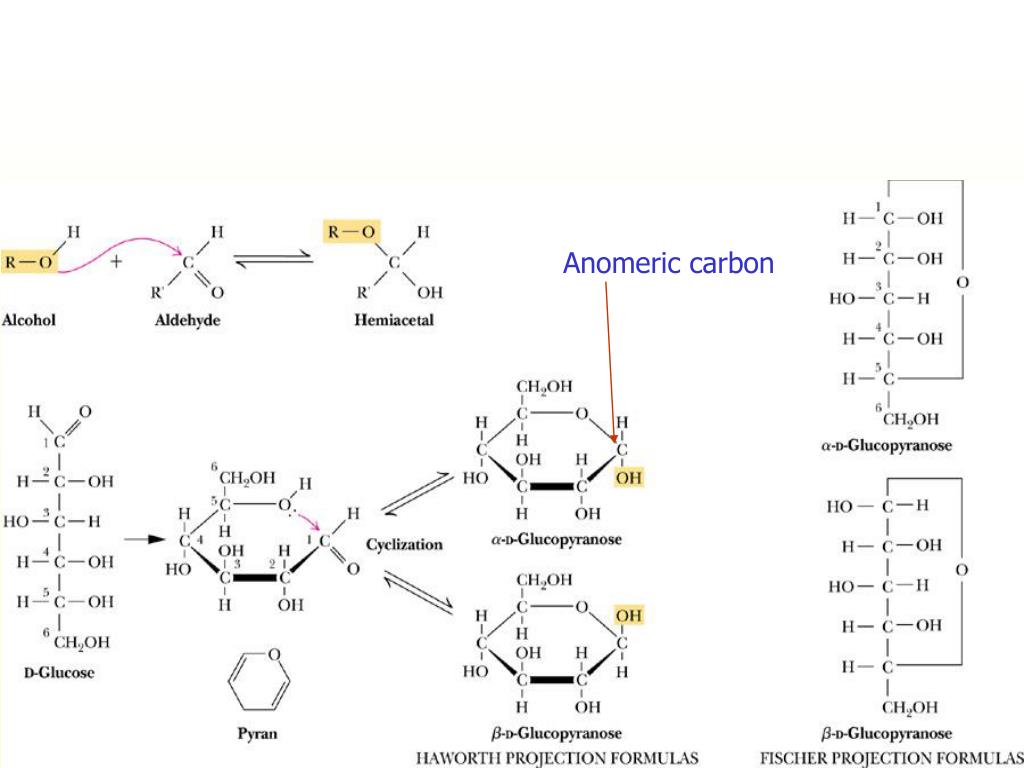
So, when I say that we’re dealing with a hexose, that doesn’t mean much except for the fact that the molecule contains 6 carbons. This list includes glucose, galactose, fructose, mannose, etc. For instance, there are 24 different hexoses (12 of which exist in nature). This type of a name, however, doesn’t tell us the exact nature of the molecule. For instance, the glucose is an example of a hexose because it has six carbons in the molecule. For instance, a triose is a carbohydrate with 3 carbons, while hexose is a carbohydrate with 6 carbons in the molecule. We use the greek numerals to call the number, aka tri-, tetra-, penta-, hexa-, and add the ending -ose to denote that it’s a carbohydrate. In this way, the anomers of Galactose can be differentiated.The simplest carbohydrate has 3 carbons. If the hydroxyl group is in the equatorial position, it is said to be beta Galactose. If the hydroxyl group in this anomeric carbon is in the axial position, it is said to be alpha Galactose. In chair form, the anomeric carbon is the carbon attached to the right side of the oxygen atom in the hexagonal ring. This is also applicable to the chair conformation of galactose. At the same time, if this hydroxyl group is attached above the ring, it is a beta Galactose. In this molecule, if this hydroxyl group is placed below the ring, the molecule is alpha Galactose. In the hexagonal ring of Galactose, the carbon which is placed at the right side of the oxygen is the anomeric carbon. From the position of the hydroxyl groups present in the ring its alpha and beta form can be differentiated. The anomers of Galactose can be understood from this conformation. Arrangement of the functional groups can be thus be clear from this conformation of galactose. In this structure, the thicker lines depict the bonds that are closer to the viewer. Galactose is drawn in cyclic structure which is referred as its Haworth projection. Galactose is an example of monosaccharides. This can be generally named as alpha-fructofuranose and beta-fructofuranose. In this way, the anomers of fructose can be identified. If the hydroxyl group in this anomeric carbon is in the axial position, it is said to be alpha fructose and if the hydroxyl group is in the equatorial position, it is said to be beta fructose. In chair form, the anomeric carbon is the carbon present on the right side of the oxygen atom in the pentagonal ring. This is also applicable to the chair conformation of fructose. In this molecule, if this hydroxyl group is placed below the ring, the molecule is alpha fructose and if this hydroxyl group is attached above the ring, it is a beta fructose. In the pentagonal ring of fructose, the carbon which is placed in the right side of the oxygen is considered as the anomeric carbon. The alpha and beta fructose can be identified from the orientation of the hydroxyl group in the anomeric carbon. As the carbonyl carbon is in the second position, this is the anomeric carbon. It can be drawn in the Haworth projection. In this way, the anomers of glucose can be differentiated.įructose is another monosaccharide with a ketone group in the molecule. If the hydroxyl group is in the equatorial position, it is said to be beta glucose. If the hydroxyl group in this anomeric carbon is in the axial position, it is said to be alpha glucose. In chair form, the anomeric carbon is the carbon attached in the right side of the oxygen atom in the hexagonal ring. This is also applicable to the chair conformation of glucose. On the other hand, if this hydroxyl group is attached above the ring, it is a beta glucose. If the hydroxyl group attached to the anomeric carbon is placed below the ring, the molecule is alpha glucose. In the hexagonal ring of glucose, the carbon atom placed in the right side of the oxygen, is the anomeric carbon. The anomers of glucose can be understood from this conformation. Arrangement of the functional groups can thus be clear from this conformation of glucose. In this structure, the thicker lines depict the bonds which are closer to the viewer. Glucose is drawn in cyclic structure which is referred as its Haworth projection. Glucose is an example of monosaccharides.


 0 kommentar(er)
0 kommentar(er)
